Published on:
24. June 2024
Basic UDI-DI and its assignment
The Basic UDI-DI is an important key in product documentation (e.g. certificates, technical documentation, vigilance notifications and PSUR, SS(C)P, etc.) and also serves as an access key to the product-related information in the European database for medical devices (EUDAMED). The Basic UDI-DI is a useful way of grouping several similar variants of a medical device.
Essential requirements for defining the Basic UDI-DI are listed in MDCG 2018-1 (currently Rev. 4). Only MDR requirements are addressed, but the guidance document is considered applicable for Regulation (EU) 2017/746 as well.These requirements must be taken into account:
The following data elements must be the same for all devices that are grouped together.
Requirements for grouping under the same Basic UDI-DI:
- Same manufacturer (name, address, SRN)
- Same risk class (in particular with regarding implantability, active medical device, special components such as drug component, materials of animal origin or substances, but also measuring function or whether it is a reusable surgical element)
- Same Medical Device Nomenclature Code (EMDN Code) – at least up to the fourth digit
- Same intended purpose
- Listed on the same product certificate, PSUR, SSCP (MP) or SSP (IVD)
- Same technical documentation (if applicable)
- Same essential design and manufacturing characteristics (if applicable)
For IVD, one Basic UDI-DI is assigned in particular for language and distribution variants (e.g. tests for self-testing in different countries for distributors with country-specific labelling) as well as packaging variants of the same product (e.g. 96-well or 128-well plates, multiple packaging).
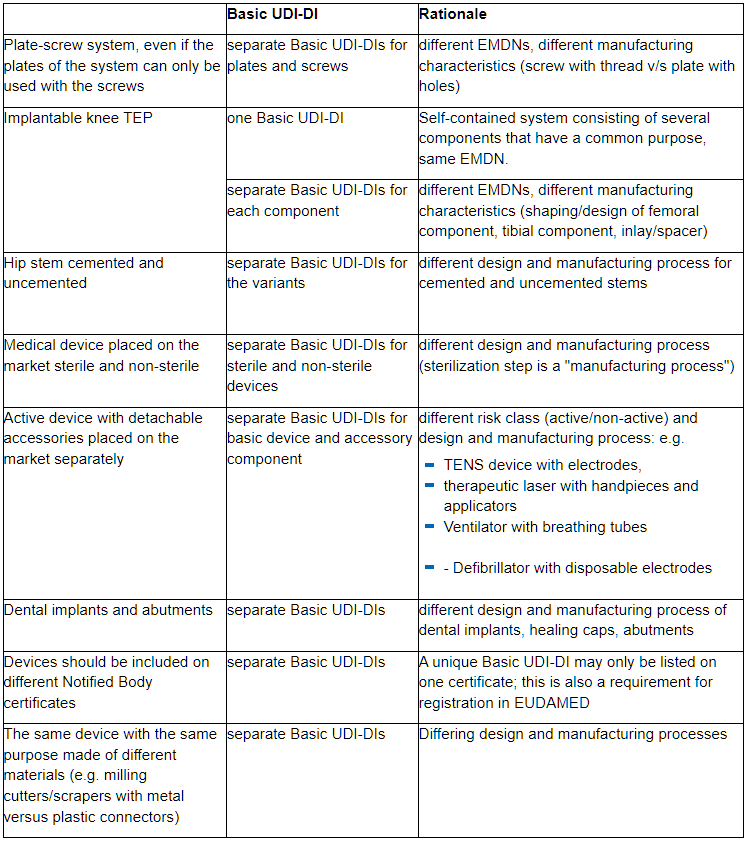
In particular with regard to the same essential design and manufacturing characteristics, we would like to point out some aspects that typically lead to different Basic UDI-DIs for medical devices: